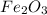Given the balanced equation representing a reaction:
fe2o3 + 2al--> al2o3 + 2fe
dur...

Chemistry, 24.01.2020 14:31 goverton101
Given the balanced equation representing a reaction:
fe2o3 + 2al--> al2o3 + 2fe
during this reaction, the oxidation number of fe changes from
(1) +2 to 0 as electrons are transferred
(2) +2 to 0 as protons are transferred
(3) +3 to 0 as electrons are transferred
(4) +3 to 0 as protons are transferred

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1


Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
Questions

Business, 12.04.2020 20:28

Mathematics, 12.04.2020 20:28


Advanced Placement (AP), 12.04.2020 20:48



History, 12.04.2020 20:48







Computers and Technology, 12.04.2020 20:49

Mathematics, 12.04.2020 20:49



History, 12.04.2020 20:49


Mathematics, 12.04.2020 20:49