
Oxygen gas can be prepared by heating potassium chlorate according to the following equation: 2kclo3(s)2kcl(s) + 3o2(g) the product gas, o2, is collected over water at a temperature of 25 °c and a pressure of 749 mm hg. if the wet o2 gas formed occupies a volume of 5.76 l, the number of moles of kclo3 reacted was mol. the vapor pressure of water is 23.8 mm hg at 25 °c.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1
You know the right answer?
Oxygen gas can be prepared by heating potassium chlorate according to the following equation: 2kclo...
Questions

Mathematics, 10.02.2021 04:50

Mathematics, 10.02.2021 04:50

History, 10.02.2021 04:50

Mathematics, 10.02.2021 04:50


Mathematics, 10.02.2021 04:50

Mathematics, 10.02.2021 04:50


Mathematics, 10.02.2021 04:50

Mathematics, 10.02.2021 04:50



Mathematics, 10.02.2021 04:50


Biology, 10.02.2021 04:50

Mathematics, 10.02.2021 04:50



English, 10.02.2021 04:50

Social Studies, 10.02.2021 04:50

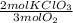 = 0,15 mol of KClO₃
= 0,15 mol of KClO₃

