
Chemistry, 18.09.2019 07:30 Lilbre9444
Asample of a compound contains 65.4 grams of zinc, 12.0 grams of carbon, and 48.0 grams of oxygen. what is the mole ratio of zinc to carbon
to oxygen in this compound?
(1) 1: 1: 2 (3) 1: 4: 6
(2) 1: 1: 3 (4) 5: 1: 4

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2


Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

Chemistry, 23.06.2019 07:30
In the diagram here that represents the reaction, which reactant, a or b, is the limiting reagent?
Answers: 1
You know the right answer?
Asample of a compound contains 65.4 grams of zinc, 12.0 grams of carbon, and 48.0 grams of oxygen. w...
Questions

Biology, 10.03.2021 19:20

Mathematics, 10.03.2021 19:20

Mathematics, 10.03.2021 19:20


Mathematics, 10.03.2021 19:20

English, 10.03.2021 19:20


Arts, 10.03.2021 19:20


Mathematics, 10.03.2021 19:20

Advanced Placement (AP), 10.03.2021 19:20

Mathematics, 10.03.2021 19:20


Mathematics, 10.03.2021 19:20

English, 10.03.2021 19:20

Mathematics, 10.03.2021 19:20

Mathematics, 10.03.2021 19:20


Business, 10.03.2021 19:20

Mathematics, 10.03.2021 19:20

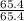 = 1
= 1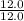 = 1
= 1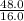 = 3
= 3

