
Chemistry, 01.11.2019 22:31 haleybain6353
Robert places a piece of magnesium metal (mg) into a test tube of hydrochloric acid (hcl). the magnesium begins to bubble and fizz, releasing hydrogen gas (h2) and the test tube gets warm. which equation represents the reaction robert observed?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1
You know the right answer?
Robert places a piece of magnesium metal (mg) into a test tube of hydrochloric acid (hcl). the magne...
Questions

Mathematics, 26.09.2019 11:50


Biology, 26.09.2019 11:50

Mathematics, 26.09.2019 11:50

Mathematics, 26.09.2019 11:50

Social Studies, 26.09.2019 11:50

English, 26.09.2019 11:50



English, 26.09.2019 11:50

History, 26.09.2019 11:50


History, 26.09.2019 11:50


History, 26.09.2019 11:50

Geography, 26.09.2019 11:50


Mathematics, 26.09.2019 11:50


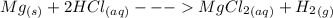
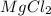 and 1 mole
and 1 mole 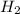 gas. The balanced equation for the reaction Robert observed is as follows:
gas. The balanced equation for the reaction Robert observed is as follows:

