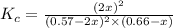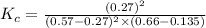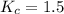Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3

Chemistry, 23.06.2019 04:00
The movement of tectonic plates and in two locations is described below: location a: tectonic played push together location b: tectonic plates push apart
Answers: 1

Chemistry, 23.06.2019 06:30
Achemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 no(g) + cl2(g) < => 2 nocl(g) kp = 2 x 10^(-6)he fills a reaction vessel at this temperature with 13. atm of nitrogen monoxide gas and 12. atm of chlorine gas. use this data to answer the questions: a. can you predict the equilibrium pressure of noci, using only the tools available to you within aleks? y/nb. if you said yes, then enter the equilibrium pressure of nocl at right. round your answer to 1 significant digit.
Answers: 1
You know the right answer?
Consider the reaction between no and cl2 to form nocl: 2no(g)+cl2(g)⇌2nocl(g) a reaction mixture at...
Questions


Chemistry, 03.12.2021 01:00


Chemistry, 03.12.2021 01:00

English, 03.12.2021 01:00




Mathematics, 03.12.2021 01:00

Chemistry, 03.12.2021 01:00


History, 03.12.2021 01:00



Mathematics, 03.12.2021 01:00

Biology, 03.12.2021 01:00

Physics, 03.12.2021 01:00


Mathematics, 03.12.2021 01:00

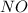 = 0.57 M
= 0.57 M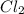 = 0.66 M
= 0.66 M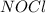 = 0.27 M
= 0.27 M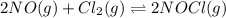
![K_c=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0356/4959/56950.png)