
Chemistry, 02.11.2019 04:31 samiller30
The maximum size the balloon can get before popping (or flying off the flask) is 6.00 l at 1.00 atm of pressure and a temperature of 45°c. what maximum concentration of hydrochloric acid can be used (to just keep the balloon from popping) if 65.0 ml of hcl is added to 9.00 g of magnesium?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
You know the right answer?
The maximum size the balloon can get before popping (or flying off the flask) is 6.00 l at 1.00 atm...
Questions





Mathematics, 30.07.2019 21:10

Mathematics, 30.07.2019 21:10

Mathematics, 30.07.2019 21:10



Geography, 30.07.2019 21:10

Mathematics, 30.07.2019 21:10



Mathematics, 30.07.2019 21:10

Mathematics, 30.07.2019 21:10


Mathematics, 30.07.2019 21:10

Mathematics, 30.07.2019 21:10


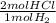 = 0.460 mol HCl
= 0.460 mol HCl

