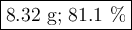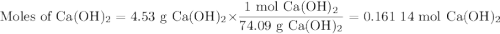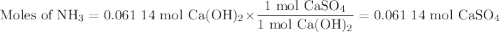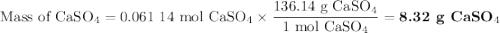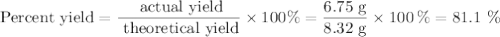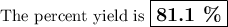For the following reaction, 4.53 grams of calcium hydroxide are mixed with excess sulfuric acid. the reaction yields 6.75 grams of calcium sulfate.
sulfuric acid (aq) + calcium hydroxide (s) calcium sulfate (s) + water (l)
what is the theoretical yield of calcium sulfate? (grams)
what is the percent yield for this reaction? (%)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1

Chemistry, 23.06.2019 07:40
Did the detergents containing enzymes work better at removing stains than those containing no enzyme? why or why not?
Answers: 2
You know the right answer?
For the following reaction, 4.53 grams of calcium hydroxide are mixed with excess sulfuric acid. the...
Questions


Mathematics, 17.07.2019 23:20

Mathematics, 17.07.2019 23:20

Biology, 17.07.2019 23:20



Mathematics, 17.07.2019 23:20