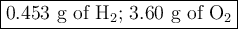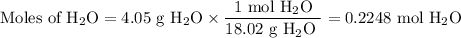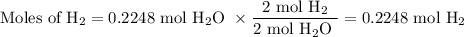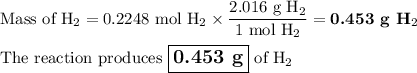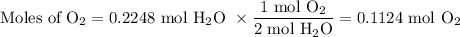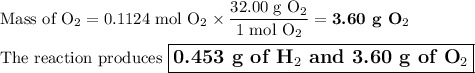3. for the following reaction, calculate how many grams of each product are formed when 4.05 g
...


Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 23.06.2019 03:00
A0.100-kilogram apple hangs in a tree 1.50 meter above the ground. ignore frictional effects, the total mechanical energy of the apples is
Answers: 1
You know the right answer?
Questions








Social Studies, 06.04.2021 06:40

Mathematics, 06.04.2021 06:40



Mathematics, 06.04.2021 06:40

Mathematics, 06.04.2021 06:40


Mathematics, 06.04.2021 06:40

Mathematics, 06.04.2021 06:40



Physics, 06.04.2021 06:40

Social Studies, 06.04.2021 06:40