

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:00
If a reaction has g of -136kj at 110°c, will it be spontaneous at this temperature (110°c)? yes or no
Answers: 2

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 23.06.2019 01:00
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
You know the right answer?
The molar concentrations for the reactants and products at equilibrium are found to be [hcl]=0.80 m,...
Questions

Mathematics, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01


Mathematics, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01


Mathematics, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01


Computers and Technology, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01


English, 20.09.2020 17:01


Geography, 20.09.2020 17:01


Mathematics, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01

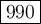
![K_{\text{eq}} = \dfrac{\text{[Cl$_{2}$]$^{2}$[H$_{2}$O]$^{2}$}}{\text{[HCl]$^{4}$[O$_{2}$]}} = \dfrac{3.0^{2}\times3.0^{2}}{0.80^{4}\times 0.20} = \mathbf{990}\\\\\text{The $K_{\text{eq}}$ value is $\large \boxed{\mathbf{990}}$}](/tpl/images/0358/6077/a43ca.png)


