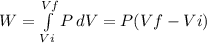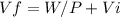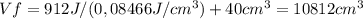Consider a mixture of air and gasoline vapor in a cylinder with a piston. the original volume is 40. cm3. if the combustion of this mixture releases 912 j of energy, to what volume will the gases expand against a constant pressure of 635 torr if all the energy of combustion is converted into work to push back the piston?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Urea, co(nh2)2, is manufactured on a large scale for use in producing urea-formaldehyde plastics and as a fertilizer. what is the maximum mass of urea that can be manufactured from the co2 produced by combustion of 1.00 x 104 grams of co2?
Answers: 1


Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1
You know the right answer?
Consider a mixture of air and gasoline vapor in a cylinder with a piston. the original volume is 40....
Questions

Chemistry, 10.11.2020 02:30

Computers and Technology, 10.11.2020 02:30

History, 10.11.2020 02:30


History, 10.11.2020 02:30

Advanced Placement (AP), 10.11.2020 02:30

Health, 10.11.2020 02:30

English, 10.11.2020 02:30



Mathematics, 10.11.2020 02:30

Mathematics, 10.11.2020 02:30



English, 10.11.2020 02:30


Business, 10.11.2020 02:30

English, 10.11.2020 02:30


Law, 10.11.2020 02:30