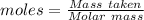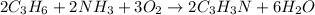Chemistry, 04.11.2019 21:31 justhereforanswers13
Acrylonitrile c3h3n is the starting material for the production of a type of widely used plastic called acrylics. acrylonitrile can be prepared from propylene, c3h6 by reaction with ammonia and oxygen. find the percent yield of acrylonitrile if the reaction yield is 91g when 84 g of propylene and 34 g of ammonia are reacted

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
Acrylonitrile c3h3n is the starting material for the production of a type of widely used plastic cal...
Questions

English, 10.10.2019 05:00

Social Studies, 10.10.2019 05:00

History, 10.10.2019 05:00




English, 10.10.2019 05:00

Biology, 10.10.2019 05:00

Mathematics, 10.10.2019 05:00

History, 10.10.2019 05:00



Chemistry, 10.10.2019 05:00

Mathematics, 10.10.2019 05:00

Spanish, 10.10.2019 05:00


Biology, 10.10.2019 05:00

History, 10.10.2019 05:00

Chemistry, 10.10.2019 05:00

English, 10.10.2019 05:00