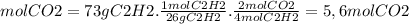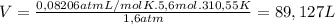Chemistry, 04.11.2019 22:31 kenleighbrooke67
Ethyne gas combusts with oxygen gas according to the following reaction: calculate the volume, in ml of co2 produced when 73 g of c2h2 react at 37.4 °c and 1.6 atm. (r = 0.08206 l atm/mol k) latex: 2\: c_2h_2\left(g\right)\: +\: 5o_2\left(g\right)\: \longrightarrow\: 4\: co_2\left(g\right)\: +2\: h_2o\left(l\right)\:

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
Ethyne gas combusts with oxygen gas according to the following reaction: calculate the volume, in m...
Questions


Biology, 13.10.2019 01:30

History, 13.10.2019 01:30



Mathematics, 13.10.2019 01:30

Biology, 13.10.2019 01:30


Mathematics, 13.10.2019 01:30





History, 13.10.2019 01:30

Arts, 13.10.2019 01:30


History, 13.10.2019 01:30

Chemistry, 13.10.2019 01:30

Business, 13.10.2019 01:30

Biology, 13.10.2019 01:30