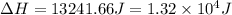Chemistry, 04.11.2019 23:31 mentatmenot
Calculate the enthalpy change (in joules) involved in converting 5.00 grams of water at 14.0 °c to steam at 115 °c under a constant pressure of 1 atm. the specific heats of ice, liquid water, and steam are, respectively, 2.03, 4.18, 1.84 j/g-k and for water δhfusion = 6.01 kj/mole and δhvap = 40.67 kj/mole
a. 1.32x10^4 j
b. 2.05 x 10^5 j
c. 195x 10^3 j
d. 1.94 x 10^3 j

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1


Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
Calculate the enthalpy change (in joules) involved in converting 5.00 grams of water at 14.0 °c to s...
Questions



Chemistry, 30.03.2021 08:50



Physics, 30.03.2021 08:50



Mathematics, 30.03.2021 08:50

Mathematics, 30.03.2021 08:50

Health, 30.03.2021 09:00


Spanish, 30.03.2021 09:00

Mathematics, 30.03.2021 09:00

Spanish, 30.03.2021 09:00

English, 30.03.2021 09:00


Mathematics, 30.03.2021 09:00


Mathematics, 30.03.2021 09:00

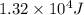
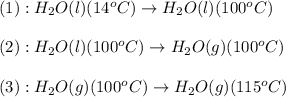
![\Delta H=[m\times c_{p,l}\times (T_{final}-T_{initial})]+n\times \Delta H_{vap}+[m\times c_{p,g}\times (T_{final}-T_{initial})]](/tpl/images/0359/4713/cc64b.png)
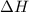 = enthalpy change or heat required = ?
= enthalpy change or heat required = ?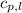 = specific heat of liquid water =
= specific heat of liquid water = 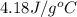
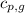 = specific heat of liquid water =
= specific heat of liquid water = 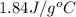
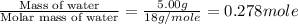
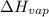 = enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole
= enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole![\Delta H=[5.00g\times 4.18J/g^oC\times (100-14)^oC]+0.278mole\times 40670J/mole+[5.00g\times 1.84J/g^oC\times (115-100)^oC]](/tpl/images/0359/4713/26444.png)