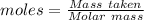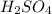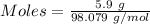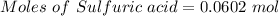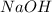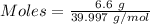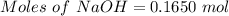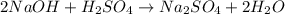Chemistry, 05.11.2019 00:31 safiyabrowne7286
Aqueous sulfuric acid (h2so4) reacts with solid sodium hydroxide (naoh) to produce aqeous sodium sulfate (na2so4) and liquid water (h2o). what is the theoretical yield of water formed from the reaction of 5.9 g of sulfuric acid and 6.6 g of sodium hydroxide?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 23.06.2019 05:30
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3
You know the right answer?
Aqueous sulfuric acid (h2so4) reacts with solid sodium hydroxide (naoh) to produce aqeous sodium sul...
Questions




Physics, 14.07.2020 02:01


Computers and Technology, 14.07.2020 02:01


History, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01

Biology, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01

Social Studies, 14.07.2020 02:01

History, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01

English, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01