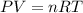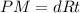Chemistry, 05.11.2019 00:31 Imamdiallo18
Assuming ideal behavior, one mole krypton gas is released into flexible container at stp. what is the density of krypton gas in this container at stp? at stp one mole of any gas occupies 22.4 l. (r = 0.08206 l • atm/k • mol) atomic mass of krypton is 83.8 amu. select one: a. 0.0561 g/l b. 0.0176 g/l c. 3.74 g/l d. 181 g/l e. 1.78 g/l

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 23.06.2019 04:20
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1

You know the right answer?
Assuming ideal behavior, one mole krypton gas is released into flexible container at stp. what is th...
Questions

Mathematics, 18.02.2021 03:00


Mathematics, 18.02.2021 03:00


Chemistry, 18.02.2021 03:00



Mathematics, 18.02.2021 03:00

History, 18.02.2021 03:00


Mathematics, 18.02.2021 03:00


Mathematics, 18.02.2021 03:00

History, 18.02.2021 03:00

Chemistry, 18.02.2021 03:00

Computers and Technology, 18.02.2021 03:00

Mathematics, 18.02.2021 03:00

Mathematics, 18.02.2021 03:00