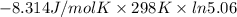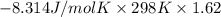Chemistry, 05.11.2019 00:31 jessemartinez1
For the reaction a(g) + 2 b(g) ↔ c(g) the initial partial pressures of gases a, b, and c are all 0.109 atm. once equilibrium has been established, it is found that pc = 0.047 atm. what is δg° for this reaction (in kj/mol) at 25°c?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 22.06.2019 04:00
Mitosis is a type of cell division that produces cells that are identical to the parent cell. meiosis is a different type of cell division that produces cells that carry have a genetic material of the parent cell. based on the information provided how do the purpose of mitosis and meiosis differ
Answers: 3

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

You know the right answer?
For the reaction a(g) + 2 b(g) ↔ c(g) the initial partial pressures of gases a, b, and c are all 0.1...
Questions

Chemistry, 06.06.2020 02:04


Mathematics, 06.06.2020 02:04

History, 06.06.2020 02:04

Mathematics, 06.06.2020 02:04

Mathematics, 06.06.2020 02:04














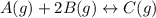
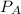 = 0.109 atm,
= 0.109 atm, 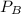 = 0.109 atm,
= 0.109 atm,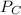 = 0.109 atm
= 0.109 atm![[0.109 + (2 \times 0.062)]](/tpl/images/0359/5284/f1aca.png) atm
atm 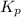 as follows.
as follows.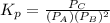
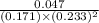
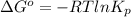
 as follows.
as follows.