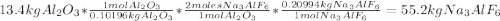Chemistry, 05.11.2019 01:31 yeetmaster7688
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. balance the equation for the synthesis of cryolite. equation: al2o3 (s) + naoh (l) + hf (g) -> na3alf6 + h2o (g) al2o3(s)+naoh(l)+hf(g)⟶na3alf6+h2o( g) if 13.4 kg of al2o3(s), 55.4 kg of naoh(l), and 55.4 kg of hf(g) react completely, how many kilograms of cryolite will be produced?

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 02:00
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

Chemistry, 23.06.2019 03:30
Ineed pls urgent 1-20 in order and fully detail step my step.
Answers: 1

Chemistry, 23.06.2019 03:30
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1
You know the right answer?
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum o...
Questions


Physics, 20.09.2020 01:01


English, 20.09.2020 01:01


Physics, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01


Business, 20.09.2020 01:01


History, 20.09.2020 01:01

History, 20.09.2020 01:01

Biology, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01


Mathematics, 20.09.2020 01:01


Physics, 20.09.2020 01:01


English, 20.09.2020 01:01

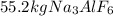
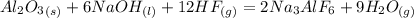
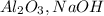 and
and 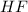
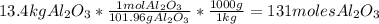
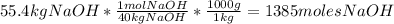
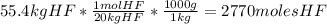
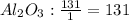
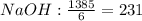
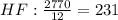
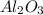 is the limiting reagent because it has the smallest number.
is the limiting reagent because it has the smallest number.