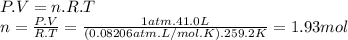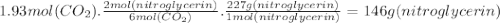Nitroglycerin is a dangerous powerful explosive that violently decomposes when it is shaken or dropped. the swedish chemist alfred nobel (1833-1896) founded the nobel prizes with a fortune he made by inventing dynamite, a mixture of nitroglycerin and inert ingredients that was safe to handle.
1. write a balanced chemical equation, including physical state symbols, for the decomposition of liquid nitroglycerin ( c3h5no33 ) into gaseous dinitrogen, gaseous dioxygen, gaseous water and gaseous carbon dioxide.
2. suppose 41.0l of carbon dioxide gas are produced by this reaction, at a temperature of −14.0°c and pressure of exactly 1atm . calculate the mass of nitroglycerin that must have reacted. round your answer to 3 significant digits. g

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:30
As a part of an experiment a student burns propane to produce carbon dioxide and water she remembers that she must follow the law conservation of matter when writing a balanced chemical equation which of these equation adheres to the law of conservation of matter
Answers: 1

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
Nitroglycerin is a dangerous powerful explosive that violently decomposes when it is shaken or dropp...
Questions




Chemistry, 02.04.2020 00:59

Mathematics, 02.04.2020 00:59

Computers and Technology, 02.04.2020 00:59


Mathematics, 02.04.2020 00:59


Mathematics, 02.04.2020 00:59


Mathematics, 02.04.2020 00:59


Mathematics, 02.04.2020 01:00

Mathematics, 02.04.2020 01:00



Mathematics, 02.04.2020 01:00