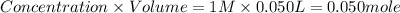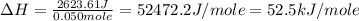Chemistry, 05.11.2019 02:31 briseno138
A50.0 ml sample of a 1.00 m solution of cuso4 is mixed with 50.0 ml of 2.00 m koh in a calorimeter. the temperature of both solutions was 20.2 degree celsious before mixing and 26.3 degree celsious after mixing . the heat capacity of the calorimeter is 12.1 j/k. from the data, calculate delta hfor the process
cuso4(1 m)+2koh(2 (oh)2(s)+k2so4(0.5 m)
assume that the specific heat and density of the solution after mixing are the same as those of pure water and that the volumes are additive.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

You know the right answer?
A50.0 ml sample of a 1.00 m solution of cuso4 is mixed with 50.0 ml of 2.00 m koh in a calorimeter....
Questions

History, 09.10.2019 10:30

Mathematics, 09.10.2019 10:30


English, 09.10.2019 10:30



Mathematics, 09.10.2019 10:30


Mathematics, 09.10.2019 10:30

Spanish, 09.10.2019 10:30

Geography, 09.10.2019 10:30

Health, 09.10.2019 10:30

Mathematics, 09.10.2019 10:30


Physics, 09.10.2019 10:30

Arts, 09.10.2019 10:30


Mathematics, 09.10.2019 10:30


Geography, 09.10.2019 10:30

![q=[q_1+q_2]](/tpl/images/0359/7670/341bc.png)
![q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0359/7670/1d50b.png)
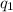 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter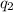 = heat absorbed by the solution
= heat absorbed by the solution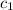 = specific heat of calorimeter =
= specific heat of calorimeter = 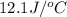
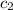 = specific heat of water =
= specific heat of water = 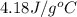
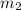 = mass of water or solution =
= mass of water or solution = 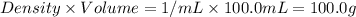
 = change in temperature =
= change in temperature = 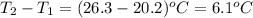
![q=[(12.1J/^oC\times 6.1^oC)+(100.0g\times 4.18J/g^oC\times 6.1^oC)]](/tpl/images/0359/7670/91dfe.png)
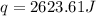
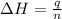
 = enthalpy change = ?
= enthalpy change = ?