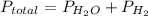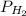Chemistry, 05.11.2019 02:31 jitosfc916
If the vapor pressure of water ( ) at 20.0°c is 17.5 mm hg, and the atmospheric pressure measured by a barometer (
) at 20.0°c is 17.5 mm hg, and the atmospheric pressure measured by a barometer ( ) was 757.3 mm hg, what is the partial pressure of h₂ gas (
) was 757.3 mm hg, what is the partial pressure of h₂ gas ( ) in the gas mixture in a buret? (use dalton’s law of partial pressures to solve for
) in the gas mixture in a buret? (use dalton’s law of partial pressures to solve for  )
)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

You know the right answer?
If the vapor pressure of water ([tex]p_{h_2o}[/tex]) at 20.0°c is 17.5 mm hg, and the atmospheric pr...
Questions

Mathematics, 26.05.2021 23:10

Mathematics, 26.05.2021 23:10

Spanish, 26.05.2021 23:10





English, 26.05.2021 23:10








Mathematics, 26.05.2021 23:10


Physics, 26.05.2021 23:10

Mathematics, 26.05.2021 23:10

History, 26.05.2021 23:10