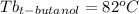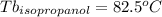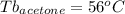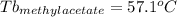Chemistry, 05.11.2019 02:31 Tweektweak
Astudent is given an unknown, clear, colorless liquid at room temperature. the student measures the density, melting point, and boiling point of the liquid. what would the student conclude if he or she found out that the unknown had a density of .79 g/cm3 and a boiling point is 82.05°c? a the substance is t-butanol b the substance is isopropanol c the substance is acetone d the substance is methyl acetate

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3
You know the right answer?
Astudent is given an unknown, clear, colorless liquid at room temperature. the student measures the...
Questions

Biology, 11.02.2021 02:10

History, 11.02.2021 02:10

English, 11.02.2021 02:10


Mathematics, 11.02.2021 02:10


History, 11.02.2021 02:10

History, 11.02.2021 02:10


Mathematics, 11.02.2021 02:10

Business, 11.02.2021 02:10



Mathematics, 11.02.2021 02:10

Mathematics, 11.02.2021 02:10

Computers and Technology, 11.02.2021 02:10

Mathematics, 11.02.2021 02:10

Mathematics, 11.02.2021 02:10

Mathematics, 11.02.2021 02:10