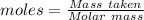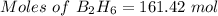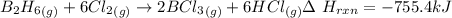Compounds of boron and hydrogen are remarkable for their unusual bonding and also for their reactivity. with the more reactive halogens, for example, diborane (b2h6) forms trihalides even at low temperatures: b2h6(g) + 6 cl2(g) → 2 bcl3(g) + 6 hcl(g) δ hrxn = −755.4 kj how much heat is released when 4.465 kg of diborane reacts? (give your answer in scientific notation.)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 23.06.2019 07:30
In a laboratory determination of the atomic weight of tin, a sample of tin is weighed in a crucible. nitric acid is added, and the reaction proceeds to give a hydrated tin(iv)oxide plus no2and h2o. the hydrated tin(iv)oxide is then heated strongly and reacts as follows: sno2.xh2o(s)sno2(s)+ xh2o(g)the sno2is finally cooled and weighed in the crucible. explain the effect on the calculated atomic weight of tin that would result from each of the following experimental errors: (a)considerable spattering occurs when the nitric acid is added to the tin.(b)the hydrated tin(iv)oxide is not heated sufficiently to change it completely to tin oxide.
Answers: 2
You know the right answer?
Compounds of boron and hydrogen are remarkable for their unusual bonding and also for their reactivi...
Questions




Mathematics, 11.07.2019 11:30

Social Studies, 11.07.2019 11:30






History, 11.07.2019 11:30

Mathematics, 11.07.2019 11:30

Mathematics, 11.07.2019 11:30



Health, 11.07.2019 11:30





 :-
:-