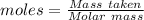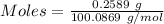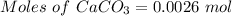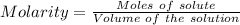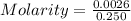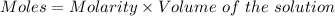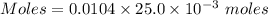A0.2589 g sample of caco3 is dissolved in 6 m hcl and the resulting solution is diluted to 250.0 ml in a volumetric flask. titration of a 25.00 ml sample of the solution requires 29.55 ml of edta to reach the eriochrome black t end point. how many moles of caco3 (cacl2) are in the 25.00 ml aliquot? select all that apply.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1
You know the right answer?
A0.2589 g sample of caco3 is dissolved in 6 m hcl and the resulting solution is diluted to 250.0 ml...
Questions

Mathematics, 07.11.2019 14:31



Health, 07.11.2019 14:31

Mathematics, 07.11.2019 14:31

English, 07.11.2019 14:31



Physics, 07.11.2019 14:31


Arts, 07.11.2019 14:31

Mathematics, 07.11.2019 14:31


Mathematics, 07.11.2019 14:31


Arts, 07.11.2019 14:31


History, 07.11.2019 14:31


Mathematics, 07.11.2019 14:31

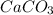 = 0.00026 moles
= 0.00026 moles