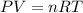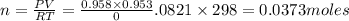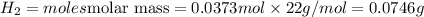Chemistry, 05.11.2019 04:31 babyface1686
The zinc within a copper-plated penny will dissolve in hydrochloric acid if the copper coating is filed down in several spots (so that the hydrochloric acid can get to the zinc). the reaction between the acid and the zinc is as follows: 2h+(aq)+zn(s)→h2(g)+zn2+(aq). when the zinc in a certain penny dissolves, the total volume of gas collected over water at 25 ∘c was 0.953 l at a total pressure of 752 mmhg .

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Nickel crystallizes in the face-centered cubic (fcc) lattice. the density of the metal is 8902 kg/m3. calculate the radius of a nickel atom.
Answers: 1


Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2
You know the right answer?
The zinc within a copper-plated penny will dissolve in hydrochloric acid if the copper coating is fi...
Questions

History, 05.01.2020 00:31

History, 05.01.2020 00:31




Physics, 05.01.2020 00:31

Mathematics, 05.01.2020 00:31




History, 05.01.2020 00:31




Geography, 05.01.2020 00:31


History, 05.01.2020 00:31

English, 05.01.2020 00:31

History, 05.01.2020 00:31

Mathematics, 05.01.2020 00:31

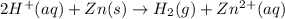
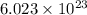 of particles.
of particles.