
Chemistry, 05.11.2019 04:31 angelapegues20097
A22.4 l high pressure reaction vessel is charged with 0.5020 mol of iron powder and 1.39 atm of oxygen gas at standard temperature. on heating, the iron and oxygen react according to the balanced reaction below. 4fe(s) + 3o2(g) → 2fe2o3(s) after the reaction vessel is cooled, and assuming the reaction goes to completion, what pressure of oxygen remains?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
State the formula for density in words and mathematical symbols
Answers: 2

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
You know the right answer?
A22.4 l high pressure reaction vessel is charged with 0.5020 mol of iron powder and 1.39 atm of oxyg...
Questions

Chemistry, 30.06.2019 11:30



History, 30.06.2019 11:30

Mathematics, 30.06.2019 11:30


History, 30.06.2019 11:30

Mathematics, 30.06.2019 11:30

Computers and Technology, 30.06.2019 11:30








World Languages, 30.06.2019 11:30



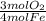 = 0.3765 mol O₂
= 0.3765 mol O₂


