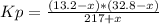An equilibrium mixture of pcl₅(g), pcl₃(g), and cl₂(g) has partial pressures of 217.0 torr, 13.2 torr, and 13.2 torr, respectively. a quantity of cl₂(g) is injected into the mixture, and the total pressure jumps to 263.0 torr. the appropriate chemical equation is pcl₃(g)+cl₂(g)↽−−⇀pcl₅(g) calculate the new partial pressures after equilibrium is reestablished.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1
You know the right answer?
An equilibrium mixture of pcl₅(g), pcl₃(g), and cl₂(g) has partial pressures of 217.0 torr, 13.2 tor...
Questions

Biology, 15.10.2019 03:10

History, 15.10.2019 03:10

Mathematics, 15.10.2019 03:10

History, 15.10.2019 03:10

History, 15.10.2019 03:10

History, 15.10.2019 03:10














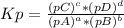 , where pX is the partial pressure of X.
, where pX is the partial pressure of X.