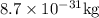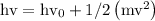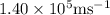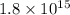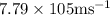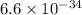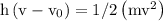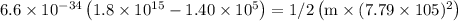Ametal with a threshold frequency of 1.40×1015
s
−1
emits an electron with a velo...


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

You know the right answer?
Questions


Mathematics, 08.12.2021 23:40


Mathematics, 08.12.2021 23:40

Social Studies, 08.12.2021 23:40

Computers and Technology, 08.12.2021 23:40

Mathematics, 08.12.2021 23:40

Mathematics, 08.12.2021 23:40

SAT, 08.12.2021 23:40


Mathematics, 08.12.2021 23:40

History, 08.12.2021 23:40


Health, 08.12.2021 23:40

Social Studies, 08.12.2021 23:40

Mathematics, 08.12.2021 23:40



History, 08.12.2021 23:40

Social Studies, 08.12.2021 23:40