Chemistry, 05.11.2019 05:31 sainijasdeep27
Three glass bulbs, joined by closed stopcocks, have the following volumes and initial pressures of the specified gases.
bulb a: 200. ml of kr(g) at 190. torr
bulb b: 400. ml of h2s(g) at 1.00 atm
bulb c: 1.00 l of n2(g) at 75.994 kpa
after both stopcocks are opened and the gases allowed to diffuse throughout, what will be the ultimate total pressure?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1
You know the right answer?
Three glass bulbs, joined by closed stopcocks, have the following volumes and initial pressures of t...
Questions


Mathematics, 01.07.2019 10:30

History, 01.07.2019 10:30

History, 01.07.2019 10:30


English, 01.07.2019 10:30

History, 01.07.2019 10:30



SAT, 01.07.2019 10:30

Mathematics, 01.07.2019 10:30

Mathematics, 01.07.2019 10:30



History, 01.07.2019 10:30

Mathematics, 01.07.2019 10:30

Physics, 01.07.2019 10:30



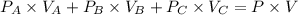
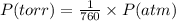
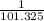 P (atm)
P (atm)
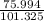 atm
atm