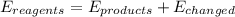Which of the statements are true? select all that apply. a) the entropy at the start of a reaction is always greater than the entropy of the products. b) the amount of usable energy resulting from a reaction is always less than the total energy available at the start of the reaction. c) the entropy of the products of a reaction is always greater than the entropy at the start of the reaction. d) the energy for a reaction equals the sum of the energy in the product plus energy released as heat and disorder.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
You know the right answer?
Which of the statements are true? select all that apply. a) the entropy at the start of a reaction...
Questions



Advanced Placement (AP), 13.02.2022 19:40

Arts, 13.02.2022 19:40

Mathematics, 13.02.2022 19:40






Mathematics, 13.02.2022 19:40

Mathematics, 13.02.2022 19:40

Mathematics, 13.02.2022 19:40


Mathematics, 13.02.2022 19:40

Mathematics, 13.02.2022 19:40

Mathematics, 13.02.2022 19:40

Mathematics, 13.02.2022 19:40