
Chemistry, 05.11.2019 22:31 realoneree
The following molecular equation represents the reaction that occurs when aqueous solutions of silver(i) nitrate and chromium(iii) bromide are combined. 3agno3 (aq) + crbr3 (aq) --> 3agbr (s) + cr(no3)3 (aq) write the balanced net ionic equation for the reaction.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2
You know the right answer?
The following molecular equation represents the reaction that occurs when aqueous solutions of silve...
Questions

Mathematics, 14.01.2021 19:00



Arts, 14.01.2021 19:00


History, 14.01.2021 19:00


Physics, 14.01.2021 19:00


History, 14.01.2021 19:00




Mathematics, 14.01.2021 19:00

World Languages, 14.01.2021 19:00

Mathematics, 14.01.2021 19:00

Mathematics, 14.01.2021 19:00


Physics, 14.01.2021 19:00

Mathematics, 14.01.2021 19:00

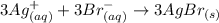
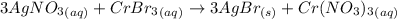
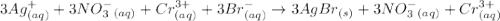
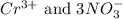 are the spectator ions.
are the spectator ions.


