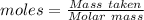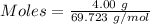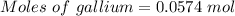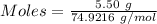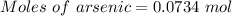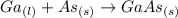Chemistry, 05.11.2019 22:31 Princess14321
Molten gallium reacts with arsenic to form the semiconductor, gallium arsenide, gaas, used in light emitting diodes and solar cells: ga(l) + as(s) → gaas(s) if 4.00 g of gallium is reacted with 5.50 g of arsenic how many grams of the excess reactant are left at the end of the reaction?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1

Chemistry, 23.06.2019 06:00
Nthis lab, you will do experiments to identify types of changes. using the question format you learned (shown above), write an investigative question that you can answer by doing these experiments
Answers: 3
You know the right answer?
Molten gallium reacts with arsenic to form the semiconductor, gallium arsenide, gaas, used in light...
Questions









Mathematics, 25.02.2020 20:21


History, 25.02.2020 20:21

English, 25.02.2020 20:21




Mathematics, 25.02.2020 20:21

English, 25.02.2020 20:21