
Avogadro constant is 6.02 1023 (the number of atoms or molecules per mole). what how much charge is there in 1 mole of electrons? how much charge is there in 1 mole of hydrogen ions (h+). remember that hydrogen consists of one electron and one proton, so hydrogen ions are just protons.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:00
How many ions that have a +1 charge will bond with an ion that has a -2 charge
Answers: 1

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1


Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2
You know the right answer?
Avogadro constant is 6.02 1023 (the number of atoms or molecules per mole). what how much charge is...
Questions



History, 10.03.2021 22:20

Mathematics, 10.03.2021 22:20

Mathematics, 10.03.2021 22:20


Business, 10.03.2021 22:20


Mathematics, 10.03.2021 22:20

History, 10.03.2021 22:20

Geography, 10.03.2021 22:20


English, 10.03.2021 22:20




Mathematics, 10.03.2021 22:20

English, 10.03.2021 22:20

Mathematics, 10.03.2021 22:20

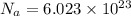
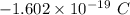
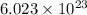 electrons =
electrons = 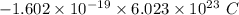 = -96488.46 C
= -96488.46 C


