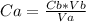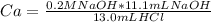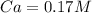Chemistry, 06.11.2019 01:31 realoneree
In a titration experiment, 13.0 ml of an aqueous hcl solution was titrated with 0.2 m naoh solution. the equivalence point in the titration was reached when 11.1 ml of the naoh solution was added. what is the molarity of the hcl solution? hcl molarity

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1


Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2
You know the right answer?
In a titration experiment, 13.0 ml of an aqueous hcl solution was titrated with 0.2 m naoh solution....
Questions

History, 09.01.2020 21:31


Mathematics, 09.01.2020 21:31



Biology, 09.01.2020 21:31



History, 09.01.2020 21:31


Chemistry, 09.01.2020 21:31

Mathematics, 09.01.2020 21:31




English, 09.01.2020 21:31