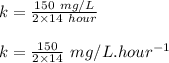Engineers do a treatment study on an industrial waste containing chemical x. in a batch reactor, the concentration of chemical x drops from 150 mg/l to 75 mg/l in 14 hours.
(a) what is the half-life?
(b) what is the decay constant if the reaction is first order?
(c) what is the decay constant if the reaction is zero order?
(d) how would you determine whether the reaction is zero or first order? if you had a set of concentration

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
Engineers do a treatment study on an industrial waste containing chemical x. in a batch reactor, the...
Questions







Mathematics, 22.01.2021 07:00


History, 22.01.2021 07:00

Mathematics, 22.01.2021 07:00


Chemistry, 22.01.2021 07:00


Mathematics, 22.01.2021 07:00

Mathematics, 22.01.2021 07:00

English, 22.01.2021 07:00

Mathematics, 22.01.2021 07:00


Mathematics, 22.01.2021 07:00

World Languages, 22.01.2021 07:00

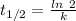
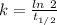
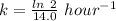
![t_{1/2}=\frac{[A]_0}{2k}](/tpl/images/0361/2925/15a8b.png)
![[A]_0](/tpl/images/0361/2925/7075c.png) is the initial concentration = 150 mg/L
is the initial concentration = 150 mg/L