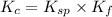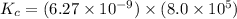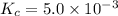Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1
You know the right answer?
Calculate the value of the equilibrium constant, kc, for the reaction cubr(s) + br−(aq) ↽⇀ cubr−2(aq...
Questions


English, 17.08.2021 16:20



Mathematics, 17.08.2021 16:20


Mathematics, 17.08.2021 16:20











Mathematics, 17.08.2021 16:20


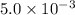
![CuBr(s)+Br^-(aq)\rightleftharpoons [CuBr_2]^-(aq)](/tpl/images/0361/4240/23bce.png)

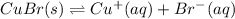
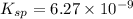
![Cu^+(aq)+2Br^-(aq)\rightleftharpoons [CuBr_2]^-(aq)](/tpl/images/0361/4240/ae2b9.png)