
Chemistry, 06.11.2019 06:31 maggie123433
The aluminum cup inside your calorimeter weighs 41.55 g. you add 59.21 g of 1.0 m acetic acid solution and 50.03 g of 1.0 m sodium hydroxide solution to the calorimeter. both solutions have an initial temperature of 19.9 oc, and the final temperature after addition is 26.8 oc. what is the molar enthalpy of neutralization, in units of kj/mol? assume that: the calorimeter is completely insulated the heat capacity of the empty calorimeter is the heat capacity of the aluminum cup: 0.903 j g-1 oc-1. the density of the two solutions is the same as that of water: 1.00 g/ml. the heat capacity of the two solutions is the same as that of water: 4.184 j g-1 oc-1.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Anurse practitioner prepares an injection of promethazine, an antihistamine used to treat allergic rhinitis. if the stock bottle is labeled 25 mg/ml and the order is a dose of 11.0 mg , how many milliliters will the nurse draw up in the syringe?
Answers: 3

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3
You know the right answer?
The aluminum cup inside your calorimeter weighs 41.55 g. you add 59.21 g of 1.0 m acetic acid soluti...
Questions





English, 08.12.2020 17:40


Advanced Placement (AP), 08.12.2020 17:40



Mathematics, 08.12.2020 17:40


English, 08.12.2020 17:40



English, 08.12.2020 17:40


Mathematics, 08.12.2020 17:40

Mathematics, 08.12.2020 17:40

Physics, 08.12.2020 17:40

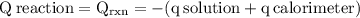
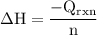
![\rm \DeltaH=-[\dfrac{- 3412.6007}{0.05003}]\\\\\Delta H=\:68211.087\dfrac{J}{mole} =68.211\dfrac{kJ}{mol}](/tpl/images/0361/7451/7b811.png)


