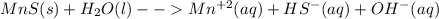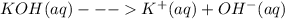Chemistry, 06.11.2019 06:31 AmyGonzalez1385
Consider the dissolution of mns in water (ksp = 3.0 × 10–14). mns(s) + h2o(l) mn2+(aq) + hs–(aq) + oh–(aq) how is the solubility of manganese(ii) sulfide affected by the addition of aqueous potassium hydroxide to the system?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

You know the right answer?
Consider the dissolution of mns in water (ksp = 3.0 × 10–14). mns(s) + h2o(l) mn2+(aq) + hs–(aq) + o...
Questions

Health, 06.10.2019 15:40






Mathematics, 06.10.2019 15:40

English, 06.10.2019 15:40

Chemistry, 06.10.2019 15:40

Mathematics, 06.10.2019 15:40


Biology, 06.10.2019 15:40


Computers and Technology, 06.10.2019 15:40

Arts, 06.10.2019 15:40


Mathematics, 06.10.2019 15:40


Biology, 06.10.2019 15:40

Biology, 06.10.2019 15:40