
Chemistry, 06.11.2019 09:31 bryan519688
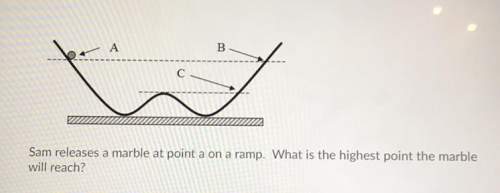
4. sam releases a marble at point a on a ramp. what is the highest point the marble will reach?
a: almost to b
b: just past b
c: point c
d: the top of the hill equal to point c
6. i take a wooden plate and an aluminum plate out of the freezer, where they have been overnight. what is true of the temperature of the plates?
a: the aluminum plate will be at a lower temperature.
b: both will be at the same temperature.
c: the wooden plate will be at a lower temperature.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 1

Chemistry, 22.06.2019 00:30
Sarah wants to know where in her garden chamomile would grow the best. she thinks chamomile will grow best in the corner of the garden that gets the most sunlight. to test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. which of the following variables will sarah need to measure to know which group of plants grew best? a. the location of the plants b. the type of plants c. the height of the plants d. the amount of water she gives the plants
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 06:00
Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. the species that is oxidized is called the reducing agent or reductant. the species that is reduced is called the oxidizing agent or oxidant. to sum up: oxidation = increase in oxidation state = loss of electrons = reducing agent reduction = decrease in oxidation state = gain of electrons = oxidizing agent part a which element is oxidized in this reaction? fe2o3+3co→2fe+3co2 enter the elemental symbol. view available hint(s) is oxidized part b which element is reduced in this reaction? 2hcl+2kmno4+3h2c2o4→6co2+2mno2+2kcl+4h2o enter the elemental symbol. view available hint(s) is reduced
Answers: 1
You know the right answer?
4. sam releases a marble at point a on a ramp. what is the highest point the marble will reach?
Questions

Mathematics, 07.11.2020 04:50

Health, 07.11.2020 04:50

English, 07.11.2020 04:50

Spanish, 07.11.2020 04:50

History, 07.11.2020 04:50




Chemistry, 07.11.2020 04:50






Mathematics, 07.11.2020 04:50

English, 07.11.2020 04:50

Biology, 07.11.2020 04:50


Biology, 07.11.2020 04:50

English, 07.11.2020 04:50



