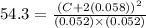Kc for the reaction of hydrogen and iodine to produce hydrogen iodide, h2(g) i2(g) ⇌ 2hi(g) is 54.3 at 430°c. determine the initial and equilibrium concentration of hi if initial concentrations of h2 and i2 are both 0.11 m and their equilibrium concentrations are both 0.052 m at 430°c.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

You know the right answer?
Kc for the reaction of hydrogen and iodine to produce hydrogen iodide, h2(g) i2(g) ⇌ 2hi(g) is 54.3...
Questions

Mathematics, 18.08.2019 12:00

Mathematics, 18.08.2019 12:00

History, 18.08.2019 12:00

Mathematics, 18.08.2019 12:00

Physics, 18.08.2019 12:00



Mathematics, 18.08.2019 12:00

English, 18.08.2019 12:00

Social Studies, 18.08.2019 12:00

English, 18.08.2019 12:00


Biology, 18.08.2019 12:00

Biology, 18.08.2019 12:00


Mathematics, 18.08.2019 12:00

Mathematics, 18.08.2019 12:00

Mathematics, 18.08.2019 12:00


Biology, 18.08.2019 12:00

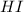 at equilibrium is, 0.27 M and 0.386 M respectively.
at equilibrium is, 0.27 M and 0.386 M respectively.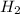 and
and 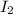 = 0.11 M
= 0.11 M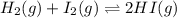
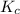 will be,
will be,![K_c=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0362/3208/62646.png)