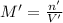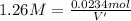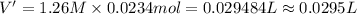Let us assume that cr(oh)3(s) is completely insoluble, which signifies that the precipitation reaction with naoh(aq) (presented in the transition) would go to completion.
cr3+(aq)+3naoh(aq) → cr(oh)3(s)+3na+(aq)
if you had a 0.600 l solution containing 0.0130 m of cr3+(aq), and you wished to add enough 1.26 m naoh(aq) toprecipitate all of the metal, what is the minimum amount of the naoh(aq) solution you would need to add? assume that the naoh(aq) solution is the only source of oh−(aq) for the precipitation.
express the volume to three significant figures and include the appropriate units.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1
You know the right answer?
Let us assume that cr(oh)3(s) is completely insoluble, which signifies that the precipitation reacti...
Questions

















Mathematics, 10.12.2019 23:31



World Languages, 10.12.2019 23:31

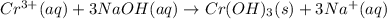
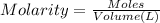
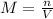
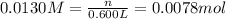
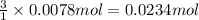 of NaOH
of NaOH