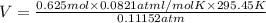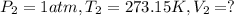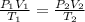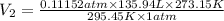Chemistry, 07.11.2019 01:31 KariSupreme
Calculate the experimental molar volume (l/mol) for an ideal gas at stp using the information that follows. a 15.0 mg piece of solid magnesium was reacted completely with hydrochloric acid in a reaction flask with a volume of 135 ml. the temperature of the reaction was 22.3 °c and the pressure of the gas produced by the reaction was 11.3 kpa. calculate the volume of hydrogen gas that would have formed in this reaction had it been conducted under standard temperature and pressure conditions (use the combined gas law). use the volume you have just determined, along with the number of moles of hydrogen gas that would have formed from 15.0 mg of magnesium reactant, to calculate the molar volume of this gas at stp

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1
You know the right answer?
Calculate the experimental molar volume (l/mol) for an ideal gas at stp using the information that f...
Questions

Biology, 30.03.2021 19:30

Social Studies, 30.03.2021 19:30





Chemistry, 30.03.2021 19:30


Mathematics, 30.03.2021 19:30

Chemistry, 30.03.2021 19:30

History, 30.03.2021 19:30

Social Studies, 30.03.2021 19:30



Mathematics, 30.03.2021 19:30

Mathematics, 30.03.2021 19:30


Mathematics, 30.03.2021 19:30

Mathematics, 30.03.2021 19:30

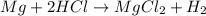
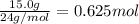
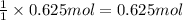 of hydrogen gas.
of hydrogen gas.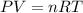 (ideal gas equation)
(ideal gas equation)