
Chemistry, 07.11.2019 01:31 kaitlynmeats
Consider the titration of 20.00 ml of 0.754 m sodium benzoate with a solution of 0.525 m nitric acid. a. calculate the equivalence volume in ml. b. calculate the ph at the equivalence point. c. calculate the ph of the solution after addition of 16.20 ml nitric acid. d. calculate the ph of the solution after addition of 39.82 ml nitric acid.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2
You know the right answer?
Consider the titration of 20.00 ml of 0.754 m sodium benzoate with a solution of 0.525 m nitric acid...
Questions




Mathematics, 20.09.2020 06:01












Mathematics, 20.09.2020 06:01

Mathematics, 20.09.2020 06:01

English, 20.09.2020 06:01


Mathematics, 20.09.2020 06:01

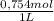 = 0,01508mol of NaBz
= 0,01508mol of NaBz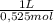 = 0,0287L = 28,7 mL
= 0,0287L = 28,7 mL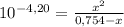
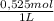 = 5,838x10⁻³moles of H⁺. The volume is 20,0mL + 39,82mL = 59,82mL ≡ 0,05982L. Thus, [H⁺] is:
= 5,838x10⁻³moles of H⁺. The volume is 20,0mL + 39,82mL = 59,82mL ≡ 0,05982L. Thus, [H⁺] is:

