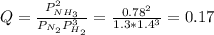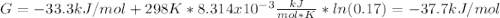In the haber process, ammonia is synthesized from nitrogen and hydrogen: n2(g)+3h2(g)→2nh3(g) δg∘ at 298k for this reaction is −33.3kj/mol. the value of δg at 298k for a reaction mixture that consists of 1.3atmn2, 1.4atmh2, and 0.78atmnh3 is kj/mol. in the haber process, ammonia is synthesized from nitrogen and hydrogen: at for this reaction is . the value of at for a reaction mixture that consists of , , and is . −4.42×103 −76.6 −37.7 −5.7 −2.13 × 103

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3
You know the right answer?
In the haber process, ammonia is synthesized from nitrogen and hydrogen: n2(g)+3h2(g)→2nh3(g) δg∘ a...
Questions

Mathematics, 29.10.2020 01:00


English, 29.10.2020 01:00



Chemistry, 29.10.2020 01:00

English, 29.10.2020 01:00

Mathematics, 29.10.2020 01:00

Biology, 29.10.2020 01:00



Mathematics, 29.10.2020 01:00


Mathematics, 29.10.2020 01:00

Mathematics, 29.10.2020 01:00


Mathematics, 29.10.2020 01:00

History, 29.10.2020 01:00


Mathematics, 29.10.2020 01:00

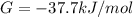
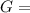 Δ
Δ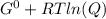
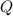 is computed via the law of mass action:
is computed via the law of mass action: