
Chemistry, 07.11.2019 02:31 whitwelllife
Pyridinium is a weak acid having a pka of 5.2. how much pyridine (the conjugate base of pyridinium) must be added to an aqueous solution of 0.100m pyridinium (assume the solution volume is constant) to make a buffer that is designed to keep the ph at 4.7?

Answers: 2
Another question on Chemistry



Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2
You know the right answer?
Pyridinium is a weak acid having a pka of 5.2. how much pyridine (the conjugate base of pyridinium)...
Questions








Mathematics, 22.10.2020 22:01

Mathematics, 22.10.2020 22:01

Mathematics, 22.10.2020 22:01

Mathematics, 22.10.2020 22:01

Chemistry, 22.10.2020 22:01


Mathematics, 22.10.2020 22:01





History, 22.10.2020 22:01

Advanced Placement (AP), 22.10.2020 22:01

![pH=pK_a+log\frac{[Conjugate\ base]}{[weak\ acid]}](/tpl/images/0363/0377/c0611.png)
![pH=pK_a+log\frac{[Py]}{PyH^+]}](/tpl/images/0363/0377/17718.png)
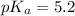
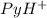 (Pyridinium)=0.100 M
(Pyridinium)=0.100 M![pH=pK_a+log\frac{[Py]}{PyH^+]}\\4.7=5.2 log\frac{[Py]}{0.100}](/tpl/images/0363/0377/de0ef.png)
![4.7-5.2=log\frac{[Py]}{0.100} \\-0.5=log\frac{[Py]}{0.100}\\\frac{[Py]}{0.100}=antilog -0.5\\\frac{[Py]}{0.100}=0.316](/tpl/images/0363/0377/5a83b.png)
![\frac{[Py]}{0.100} =0.316](/tpl/images/0363/0377/cf365.png)


