
During a spontaneous chemical reaction, it is found that δssys is less than 0. this means that group of answer choices δssurr is less than 0 and its magnitude is less than δssys. δssurr is less than 0 and its magnitude is greater than δssys. δssurr is greater than 0 and its magnitude is less than δssys. δssurr is greater than 0 and its magnitude is greater than δssys. an error has been made, as ssys is greater than 0 by necessity for a spontaneous process.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1


Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1
You know the right answer?
During a spontaneous chemical reaction, it is found that δssys is less than 0. this means that group...
Questions

Arts, 14.10.2021 22:20

Computers and Technology, 14.10.2021 22:20


Mathematics, 14.10.2021 22:20


Mathematics, 14.10.2021 22:20

Mathematics, 14.10.2021 22:20

Mathematics, 14.10.2021 22:20

Chemistry, 14.10.2021 22:20


Biology, 14.10.2021 22:20

Mathematics, 14.10.2021 22:20




English, 14.10.2021 22:30



Mathematics, 14.10.2021 22:30


 is the enthalpy change and
is the enthalpy change and  is the entropy change of the system.
is the entropy change of the system.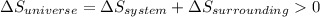
 should be greater than 0 or positive. Also,
should be greater than 0 or positive. Also, 

