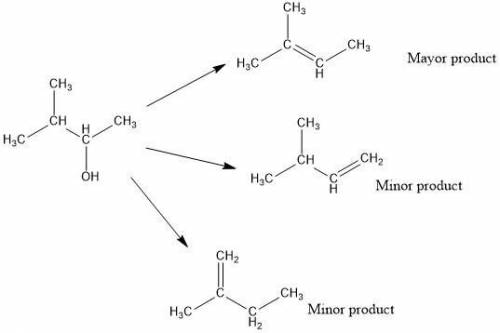
Acid-catalyzed dehydration of secondary and tertiary alcohols proceeds through an e1 mechanism. the first step is the protonation of the alcohol oxygen to form an oxonium ion. dehydration of 3-methyl-2-butanol forms one major and two minor organic products. draw the structures, including hydrogen atoms, of the three organic products of this reaction.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1


Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2
You know the right answer?
Acid-catalyzed dehydration of secondary and tertiary alcohols proceeds through an e1 mechanism. the...
Questions

Mathematics, 12.02.2021 18:50



English, 12.02.2021 18:50


SAT, 12.02.2021 18:50


Arts, 12.02.2021 18:50



Mathematics, 12.02.2021 18:50

Mathematics, 12.02.2021 18:50


Mathematics, 12.02.2021 18:50



Mathematics, 12.02.2021 18:50

Mathematics, 12.02.2021 18:50

Biology, 12.02.2021 18:50

Mathematics, 12.02.2021 18:50