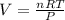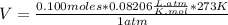Chemistry, 07.11.2019 04:31 genyjoannerubiera
Magnesium metal (0.100 mol) and hydrochloric acid (0.500 mol hcl) are combined and react to completion. what volume of hydrogen gas, measured at stp, is produced? mg(s) + 2hcl(aq) → mgcl2(aq) + h2(g) (r = 0.08206 l • atm/k • mol) select one: a. 22.4 l of h2 b. 5.60 l of h2 c. 4.48 l of h2 d. 11.2 l of h2 e. 2.24 l of h2

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1
You know the right answer?
Magnesium metal (0.100 mol) and hydrochloric acid (0.500 mol hcl) are combined and react to completi...
Questions


Mathematics, 24.03.2021 02:50


Mathematics, 24.03.2021 02:50


Mathematics, 24.03.2021 02:50

Mathematics, 24.03.2021 02:50

English, 24.03.2021 02:50

Mathematics, 24.03.2021 02:50

Mathematics, 24.03.2021 02:50


English, 24.03.2021 02:50

Mathematics, 24.03.2021 02:50


Mathematics, 24.03.2021 02:50


Computers and Technology, 24.03.2021 02:50



Mathematics, 24.03.2021 02:50

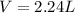 of
of 
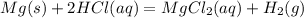
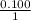
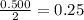
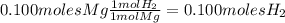
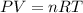 , where P is the pressure, at STP P=1 atm, V is the volume, n is the number of moles, R is a constante whose value is R=0.08206
, where P is the pressure, at STP P=1 atm, V is the volume, n is the number of moles, R is a constante whose value is R=0.08206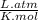 , and T is the temperature, at STP T=273K
, and T is the temperature, at STP T=273K