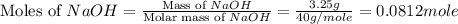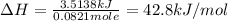Chemistry, 07.11.2019 05:31 heybrothwrlogan
When a 3.25 g sample of solid sodium hydroxide was dissolved in a calorimeter in 100.0 g of water, the temperature rose from 23.9 °c to 32.0 °c. calculate ∆h (in kj/mol) for the solution process: naoh (s) → na+ (aq) + oh- (aq)
use a calorimeter heat capacity of ccal = 15.8 j/°c

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:10
Which statement is true about the part of the electromagnetic spectrum that human eyes can detect? it contains only the colors of the rainbow and television waves. o it is divided into seven ranges of wavelengths. it contains ultraviolet, visible, and infrared light. it is divided into nine ranges of wavelengths.
Answers: 2

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1


Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3
You know the right answer?
When a 3.25 g sample of solid sodium hydroxide was dissolved in a calorimeter in 100.0 g of water, t...
Questions




Computers and Technology, 27.12.2019 00:31





Computers and Technology, 27.12.2019 00:31











![q=[q_1+q_2]](/tpl/images/0363/3965/341bc.png)
![q=[c_1\times \Delta T+m\times c_2\times \Delta T]](/tpl/images/0363/3965/21bf4.png)
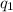 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter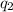 = heat absorbed by the water
= heat absorbed by the water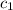 = specific heat of calorimeter =
= specific heat of calorimeter = 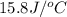
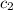 = specific heat of water =
= specific heat of water = 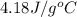
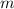 = mass of water = 100.0 g
= mass of water = 100.0 g = change in temperature =
= change in temperature = 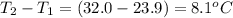
![q=[(15.8J/^oC\times 8.1^oC)+(100.0g\times 4.18J/g^oC\times 8.1^oC)]](/tpl/images/0363/3965/4042f.png)
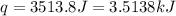 (1 kJ = 1000 J)
(1 kJ = 1000 J)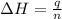
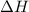 = enthalpy change = ?
= enthalpy change = ?