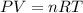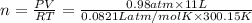Chemistry, 07.11.2019 05:31 mckleinrivero
Amaterials scientist has created an alloy containing aluminum, copper, and zinc, and wants to determine the percent composition of the alloy. the scientist takes a 13.039 g sample of the alloy and reacts it with concentrated hcl . the reaction converts all of the aluminum and zinc in the alloy to aluminum chloride and zinc chloride in addition to producing hydrogen gas. the copper does not react with the hcl . upon completion of the reaction, a total of 11 l of hydrogen gas was collected at a pressure of 744 torr and a temperature of 27.0 °c . additionally, 2.761 g of unreacted copper is recovered. calculate the mass of hydrogen gas formed from the reaction.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1


Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2
You know the right answer?
Amaterials scientist has created an alloy containing aluminum, copper, and zinc, and wants to determ...
Questions



Mathematics, 22.01.2021 19:40


Geography, 22.01.2021 19:40




Medicine, 22.01.2021 19:40

Chemistry, 22.01.2021 19:40


Business, 22.01.2021 19:40


Business, 22.01.2021 19:40





Business, 22.01.2021 19:40

Mathematics, 22.01.2021 19:40