
A0.05270.0527 g piece of metal containing an unknown amount of silver is dissolved to form 25.00 ml of solution. to the solution, 35.435.4 ml of 0.02190.0219 m kcl kcl is added, resulting in the complete precipitation of the silver ash agcl agcl . the excess cl−cl− added to the solution is then back titrated with a standard 0.02190.0219 m agno3 agno3 solution. a total of 14.3314.33 ml of the standard nano3 agno3 solution is required to reach the silver chromate end point in the mohr method. calculate the percent silver in the piece of metal.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1


Chemistry, 22.06.2019 02:00
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2
You know the right answer?
A0.05270.0527 g piece of metal containing an unknown amount of silver is dissolved to form 25.00 ml...
Questions


Mathematics, 02.10.2019 14:30

Biology, 02.10.2019 14:30


Mathematics, 02.10.2019 14:30


Mathematics, 02.10.2019 14:30


Computers and Technology, 02.10.2019 14:30


Mathematics, 02.10.2019 14:30

Biology, 02.10.2019 14:30


History, 02.10.2019 14:30

Physics, 02.10.2019 14:30

Chemistry, 02.10.2019 14:30

Mathematics, 02.10.2019 14:30


Mathematics, 02.10.2019 14:50

Mathematics, 02.10.2019 14:50

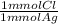 = 0.314 mmol Cl⁻
= 0.314 mmol Cl⁻

